When we talk about human milk banks in North America, we’re generally referring to the non-profit HMBANA (Human Milk Banking Association of North America) milk banks. The milk from these banks is often the safest source of lifesaving donor milk for seriously ill babies– and the only source of donor milk that many hospitals are willing to use.
The information in this article does not apply to the for-profit milk banks (e.g., Prolacta, Medolac/Mothers Milk Cooperative/Neolac) that have come into existence relatively recently, and use the donated milk to manufacture high-profit human milk-based nutritional products.
Now infants can get
all their vitamin D
from their mothers’ milk;
no drops needed with
our sponsor's
TheraNatal Lactation Complete
by THERALOGIX. Use PRC code “KELLY” for a special discount!
Why do HMBANA Milk Banks charge a processing fee for milk?

Image courtesy of the Mothers’ Milk Bank in Denver
Many mothers wonder why the non-profit HMBANA human milk banks charge a fee for milk even though the milk has been donated by mothers.
Even though the milk is donated, there are expenses that must be covered to screen donors, process the milk, distribute the milk and keep the milk banks open.
Laraine Lockhart Borman, IBCLC, with the Mothers’ Milk Bank in Denver, CO explains…
“Human Milk Banking Association of North America (HMBANA) banks do not charge for the milk itself, only for the processing of the milk and related overhead. Processing includes:
- the multi step screening of the donor, including blood tests
- donor tracking
- pasteurization
- testing and analysis of the milk
Related operational costs include:
- rental of office space
- purchase of bottles and caps
- freezers
- employee payroll (volunteers are utilized whenever possible)
“HMBANA Milk Banks are non-profit organizations making enough to keep doors open and continue to do the important work of helping to save babies’ lives. While the majority of the pasteurized human milk from the Mothers’ Milk Bank in Denver is sold to hospitals, milk depots also sell this life-saving nutrition to individuals.
“A baby with a health condition warranting the need for milk is never denied milk because of the inability to pay. Recipients always have the opportunity to apply for financial aid.”
Why do milk banks require that donors avoid regular use of most medications and herbal supplements?
HMBANA donor guidelines require that donors are:
“not regularly on most medications or herbal supplements (with the exception of prenatal vitamins, human insulin, thyroid replacement hormones, nasal sprays, asthma inhalers, topical treatments, eye drops, progestin-only or low dose estrogen birth control products; for other exceptions, please contact a milk bank for more information).”
This is because most of the milk from milk banks goes to very fragile, hospitalized babies (preterm and/or very ill), who may be much more sensitive to a medication in the milk than a healthy, full-term newborn or an older baby would be.
When a mother is providing milk to her own baby, most medications and supplements are compatible with breastfeeding. If her baby is premature or seriously ill, she will be specifically checking the safety of each medication she is taking to make sure it is not a problem for her baby (safety may vary depending on baby’s specific health issues). Since a donor’s milk may be distributed to many different babies, all with different health issues, it is not possible to tailor each donor’s medications to each baby so there are overall guidelines instead.
Why not just donate your milk mother-to-mother?
This is an option, and some mothers prefer it. Informal milk donations are generally between one (or several) specific mothers to a specific baby. Most babies receiving informal breastmilk donations are healthy and are full-term newborns or older, so there are not as many limitations when it comes to medications/supplements that the donor is taking (though this should always be discussed). It is up to the participants to ensure that the milk is handled safely, be aware of and screen for health risks, etc.
When you donate to a milk bank, your milk will be going to fragile babies whose very lives may depend on getting donated milk. Milk from these banks is usually the only donor milk available to these babies (informal donations are usually not an option in the NICU), and it is often in short supply.
Per Laraine Lockhart Borman, IBCLC, with the Mothers’ Milk Bank in Denver…
“The Mothers’ Milk Bank in Denver dispensed 330,000 ounces of milk to babies in 117 different cities last year. 95% of this milk went to hospitals for their preterm and sick babies. These are children who have been born up to 16 weeks early and may weigh a pound at birth. Because these babies are extremely fragile and will not survive without human milk, the donated milk they receive has a large impact. One ounce can feed a micro preemie in the neonatal intensive care unit (NICU) for a full day. The mothers of these tiny babies are many times ill themselves and making milk is difficult for them, or they start out with a good amount and are so stressed out with the condition of their baby that their milk supply suffers.
Why do milk banks pasteurize the milk?
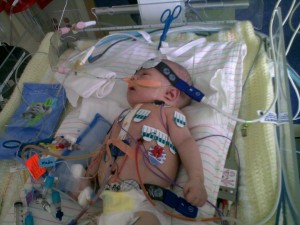
Image courtesy of the Mothers’ Milk Bank in Denver
Most of the milk from milk banks goes to very fragile, hospitalized babies (preterm and/or very ill). To protect these immune-compromised babies, the milk is pasteurized so that there is no chance of an infectious disease being transmitted through the milk. It is a myth that pasteurization destroys all the “good stuff” in human milk – most of the nutrients and immune properties remain, and there are many research studies demonstrating the health benefits of banked, pasteurized human milk.
Laraine Lockhart Borman, IBCLC, with the Mothers’ Milk Bank in Denver, CO explains…
The HMBANA Advisory Council, a panel of experts in areas of infectious disease, microbiology, neonatology, law, and other areas, determined that because human milk has the potential to be an agent of infectious disease, pasteurization would be required of all member banks due to the extremely fragile health of the babies receiving this milk. The number one priority is the health and safety of the tiny preterm infants served.
With any type of storage or treatment of human milk, there is some loss of its original components. Mother’s milk that has been frozen and stored experiences a loss of vitamin A from exposure to light and vitamin C from exposure to freezing temperatures. Pasteurization is a very gentle, controlled heating process using special equipment that kills viruses and bacteria while still maintaining 95% of everything that was originally in the milk. While a few of the immune properties are lost, there are many more that survive the pasteurization process. In addition, some beneficial enzymes are actually activated by the pasteurization process. *
Bottom line is that mom’s own milk is best. When this is not available, pasteurized human milk, obtained from a certified milk bank, can be a lifesaving safe and healthy alternative that everyone can feel good about.

Image courtesy of the Mothers’ Milk Bank in Denver
How does pasteurization affect human milk?
From “Donor Human Milk for Preterm Infants” (Wight 2001):
The benefits and concerns regarding the use of human milk for preterm infants has been recently reviewed, with more factors, actions, and interactions being discovered frequently… Protective effects of human milk on infection rates have been observed with the use of both fresh and pasteurized milk… [note: see Heiman & Schanler 2006 for a recent review]
Pasteurization (56 or 62.5°C for 30 minutes) does affect some of the nutritional, immunologic and other components of human milk. Heat treatment at 56°C (133°F) or greater for 30 minutes reliably eliminates all functional white blood cells and bacteria, inactivates human immunodeficiency virus (HIV) and human T-lymphotropic virus, and decreases the titers of other viruses, but in one study did not eliminate cytomegalovirus (CMV). Holder pasteurization [62.5°C (144°F) for 30 minutes] reliably inactivates HIV and CMV, and will eliminate or significantly decrease the titers of most other viruses.
Immunologic factors are variously affected by heat treatment. With Holder pasteurization most of the secretory IgA, bifid growth factor, and lysozyme remain (0% to 30% destroyed), lipids are unaffected, but 57% of the lactoferrin, and 34% of the IgG are destroyed. The reader is referred to a more detailed recent review (Lawrence 1999).
In general, the nutritional components are altered somewhat, resulting in slightly slower growth when compared to infants fed unpasteurized raw human milk. Holder pasteurization does not appear to influence nitrogen absorption or retention in LBW infants. Most enzymes, growth factors, vitamins, and minerals are unchanged or minimally decreased. Heat treatment of donor milk appears to foster more rapid growth of intestinal epithelial cells by inactivating heat-labile inhibitory cytokines, allowing heat-stable epidermal growth factor to act. Freezing inactivates milk cells and most viruses, but does not appear to effect the nutritional or anti-infective quality of the milk. Microwaving clearly decreases the anti-infective properties of human milk; the higher the temperature, the greater the effect.
* Research: The effect of pasteurization on the anti-infective agents of human milk
Percent activity remaining after pasteurization at 62.5°C for 30 minutes (unless otherwise noted)
| Cells | 78% macrophages | Gibbs 1977 |
| No viable cells | Liebhaber 1977 | |
| Immunoglobulins | ||
| IgA (total secretory) | 39% 81% (56°C for 30 minutes) |
Stephens 1980 |
| 67% 77% (62.5°C for 5 minutes) 90% (56°C for 30 minutes) |
Wills 1982 | |
| 67% | Liebhaber 1977 | |
| 67% 64% (72°C for 15 seconds) |
Goldsmith 1983 | |
| 78% | Morgan 1986 | |
| 80% | Ford 1977 | |
| 79% | Gibbs 1977 | |
| 84% | Goldblum 1984 | |
| 86% | Raptopoulou-Gigi 1977 | |
| 100% | Evans 1978 | |
| 150% (72°C for 15 seconds) | Goldblum 1984 | |
| Some loss (stable if 56°C for 30 min) | Welsh & May 1979 | |
| IgG | 66% | Evans 1978 |
| 86% 58% (72°C for 15 seconds) |
Goldsmith 1983 | |
| IgM | Substantial loss | Liebhaber 1977 |
| None | Goldsmith 1983 | |
| None | Ford 1977 | |
| Enzymes | ||
| Lactoperoxidase | 53% | Friend 1983 |
| Lipase | 45% | Friend 1983 |
| Protease | 27% | Friend 1983 |
| Lysozyme | 61% | Friend 1983 |
| 64% | Gibbs 1977 | |
| 67% 96% (62.5°C for 5 min) 106% (56°C for 30 minutes) |
Wills 1982 | |
| 76% | Evans 1978 | |
| 105% | Ford 1977 | |
| 393% (72°C for 15 seconds) | Goldblum 1984 | |
| Lactoferrin | 27% | Wills 1982 |
| 33% | Welsh & May 1979 | |
| 36% | Goldsmith 1983 | |
| 35% | Ford 1977 | |
| 43% | Evans 1978 | |
| 123% (72°C for 15 seconds) | Goldblum 1984 | |
| 56% | Eyres 1978 | |
| Bile salt-stimulated lipase | Lost | Wardell 1984 |
| Other | ||
| Non immunoglobulin | Stable | Laegreid 1986 |
| C1 – C9 | Destroyed | Welsh & May 1979 |
| L Bifidus growth factor | Stable | |
| Antimicrobial activity | Stable (56°C for 30 minutes) | Bullen 1972 |
| Antiprotozoal activity | Some stability | Gillin 1983 |
See also: Effect of heat treatment or storage on antimicrobial factors in human milk
More Information
Human Milk Banking and Other Donor Milk @ KellyMom
References
Bullen JJ, Rogers HJ, Leigh L. Iron-binding proteins in milk and resistance to escherichia coli infection in infants. Brit. Med. J. 1972; i:69-75.
Evans TJ, Ryley HC, Neale LM, Dodge JA, Lewarne VM. Effect of storage and heat on antimicrobial proteins in human milk. Arch. Dis. Child. 1978; 53:239-241.
Eyres R, Elliot RB, Howie RN, Farmer K. Low temperature pasteurization of human milk. N. Z. Med. J. 1978; 87:134-135.
Ford JE, Law BA, Marshall VME, Reiter B. Influence of the heat treatment of human milk on some of its protective constituents. Pediatr. 1977; 90:29-35.
Friend BA, Shahani KM, Long CA, Agel EN. Evaluation of freeze-drying, pasteurization, high-temperature heating and storage on selected enzymes, B-vitamins and lipids of mature human milk. J. Food. Prot. 1983; 46:330-334.
Gibbs JH, Fisher C, Bhattacharya S, Goddard P, Baum JD. Drip breast milk: its composition, collection and pasteurization. Early Hum. Dev. 1977; 1:227-245.
Gillin FD, Reiner DS, Wang, C-S. Human milk kills parasitic intestinal protozoa. Science. 1983; 221:1290-1292.
Goldblum RM, Dill CW, Albrecht TB, Alford ES, Garza C, Goldman AS. Rapid high-temperature treatment of human milk. J. Pediatr. 1984; 104:380-385.
Goldsmith SJ, Dickson JS, Barnhart HM, Toledo RT, Eitenmiller RR. IgA, IgG, IgM and lactoferrin contents of human milk during early lactation and the effect of processing and storage. J. Food Prot. 1983; 46:4-7.
Heiman H, Schanler RJ. Benefits of maternal and donor human milk for premature infants. Early Hum Dev. 2006 Dec;82(12):781-7. Epub 2006 Oct 20.
Laegreid A, Kolsto Otnaess A-B, Orstavik I, Carlsen KH. Neutralizing activity in human milk fractions against respiratory syncytial virus. Acta Paediatr. Scand. 1986; 75:696-701.
Lawrence RA. Storage of human milk and the influence of procedures on immunological components of human milk. Acta Paediatr Suppl. 1999 Aug;88(430):14-8. (Review)
Liebhaber M, Lewiston NJ, Asquith MT, Olds-Arroyo L, Sunshine P. Alterations of lymphocytes and of antibody content of human milk after processing. Pediatr. 1977; 91:897-900.
Morgan JN, Toledo RT, Eitenmiller RR, Barnhart NM, Maddox F. Thermal destruction of immunoglobulin A, lactoferrin, thiamin and folic acid in human milk. J. Food Sci. 1986; 51:348-351.
Raptopoulou-Gigi M, Marwick K, McClelland DBL. Antimicrobial proteins in sterilized human milk. Br. Med. J. 1977; 1:12-14.
Stephens S, Dolby JM, Montreuil J, Spik G. Differences in inhibition of the growth of commensal and enteropathogenic strains of escherichia coli by lactotransferrin and secretory immunoglobulin A isolated from human milk. Immunology. 1980; 41:597-603.
Wardell JM, Wright AJ, Bardsley WG, D’Souza SW. Bile salt-stimulated lipase and esterase activity in human milk after collection, storage and heating: Nutritional implications. Pediatr. Res. 1984;18:382-386.
Welsh JK, May JT. Anti-infective properties of breast milk. J Pediatr. 1979 Jan;94(1):1-9.
Wight NE. Donor human milk for preterm infants. J Perinatol. 2001 Jun;21(4):249-54.
Wills ME, Han VEM, Harris DA, Baum JD. Short-time low-temperature pasteurization of human milk. Early Hum. Dev. 1982; 7:71-80.
